| HOME | PYROTECHNIC LIBRARY | PYROTECHNIC CHEMICALS | PYROTECHNIC LINKS | PYROTECHNIC DATA | PYROTECHNIC SERCH ENGINE | SITE MAP | FILE INDEX |
PYROTECHNIC FILES MENUE |
|

Chemical |
Health and handling |
Sources |
Property |
Color/effect |
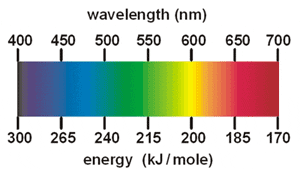
Wavelength (nm) |
Aluminum Al The most commonly used metal powder fuel to create flash powder and silvery-white sparkling effects in sparklers, gerbs, fountains, waterfalls, etc. Different types of powders, allow for wide range of possible effects, depending on particle size, shape and impurities. The finest powders (sometimes referred to as 'dark' aluminum) such as the well known 'german dark' are used mainly in flash. Fine aluminum is also used in small percentages in some rocket fuels. Coarser powders are generally used for spark effects. Depending on the particles shapes, sizes and compositions many different effects such as flitter, glitter, firefly and snowball can be achieved. |
A dust mask should be worn when working with aluminum powder. Mixtures containing nitrates and aluminum powder are prone to heating up spontaneously and may ignite, especially when wet. This is caused by the reduction o f the nitrate by aluminum, forming amides. These very basic compounds react further with aluminum powder in a very exothermic reaction that can cause spontaneous ignition. An ammonia smell is often produced in this reaction. Adding 1 to 2% boric acid to compositions containing nitrates and aluminum is common practice and will often prevent spontaneous ignition, although this should never be relied upon. It is advisable to avoid using water to bind such compositions. Red gum or shellac with alcohol or nitrocellulose lacquer are preferred binder and solvents. | Aluminum powder is sometimes sold as a pigment in (art) paint stores. This powder, known as 'aluminum bronze', is a flaky powder with a searing coating. It is quite expensive but readily available and a source for small quantities. Aluminum grit and turnings can sometimes be found in machine shops were aluminum is processed. If fine enough this can be used as is, but it can also be ball milled into flakes. These flakes are quite reactive as they have a large surface area and can be used for several effects. Sanding aluminum chunks can also make aluminum powder. I've heard of people building a machine to do this, and the results can be quite good depending on the sandi ng paper used and the set-up. Another source of usable aluminum powder is to burn tetra-paks, and then powder the resultant aluminum residue in a ball mill. | Fuel | Silver, flash | |
Ammonium Nitrate H4NO3 Not commonly used because of the problems with hygroscopicity and sensitivity with other chemicals. Ammonium nitrate is an oxidizer. It is very hygroscopic and therefore not used very often in fireworks. It finds some use in composite propellants, but performance is not as good as perchlorate-based propellants Ammonium nitrate is also an explosive in its purest form although it is an unusually insensitive one. Explosive properties become much more evident at elevated temperatures. When ammonium nitrate is fused and "boiled" to generate nitrous oxide, it has been claimed to be as sensitive as dynamite at the ~240 °C operating temperature. This exothermic reaction can run away and reach detonation velocities (without proper temperature controls). The extent of this possibility has been demonstrated several times, most notably at the Ohio Chemical plant in Montreal in 1966. Millions of pounds of relatively pure ammonium nitrate have been (accidentally) detonated when subjected to severe heat and/or shocks; see "Disasters" below. Ammonium nitrate has also found use as a solid rocket propellant, but for a while ammonium perchlorate was frequently considered preferable due to higher performance and faster burn rates. Lately, favor has been swinging back towards ammonium nitrate in rocketry, as it delivers almost as much thrust without producing an exhaust jet full of gaseous hydrochloric acid (HCl) and without the extra expense and sensitivity hazards. Fertiliser-grade ammonium nitrate (FGAN) is manufactured in more compact form, with much lower porosity, in order to achieve more stability and less sensitivity to detonation, whereas technical grade ammonium nitrate (TGAN) prills are made to be porous for better absorption of fuel and higher reactivity. |
Large masses of ammonium nitrate have been known to explode on some occasions although it is very insensitive. Smaller quantities are less likely to detonate. The risk of detonation increases when ammonium nitrate is molten or mixed with fuels such as metal powders or organic substances. Ammonium nitrate should never be mixed with chlorates as this may result in ammonium chlorate formation, possibly leading to spontaneous ignition. Mixtures of metal powders and ammonium nitrate are likely to heat up spontaneously and may ignite, especially when moist. This can sometimes be prevented by the addition of small amounts of boric acid (1 to 2%), but in general it is better to avoid these mixtures at all. The hygroscopic nature of ammonium nitrates makes this problem worse |
Ammonium nitrate solution can be prepared by neutralizing ammonia solution with nitric acid. It is advised to use a slight excess of ammonia. That is to make sure no remaining acid will be present in the ammonium nitrate obtained on evaporation and crystallization. Otherwise traces of the acid solution may be enclosed in the crystals, possibly leading to spontaneous ignition of mixtures made with it. Large quantities of ammonium nitrate can also be cheaply bought as fertilizer. In the Netherlands a fertilizer called 'kalkammonsalpeter' is sold. This consists of ammonium nitrate mixed with 'mergel', a mineral consisting mainly of calcium carbonate. The ammonium nitrate can be extracted with water. Industrial production is chemically quite simple, although technologically challenging. The acid-base reaction of ammonia with nitric acid gives a solution of ammonium nitrate: HNO3(aq) + NH3(g) → NH4NO3(aq). For industrial production, this is done using anhydrous ammonia gas and concentrated nitric acid. This reaction is violent and very exothermic. It should never be attempted by amateurs or in improvised equipment using such concentrated materials, though with plenty of dilution by water, it could be considered easy. After the solution is formed, typically at about 83% concentration, the excess water is evaporated to an ammonium nitrate (AN) content of 95 to 99.9% concentration (AN melt), depending on grade. The AN melt is then made into "prills" or small beads in a spray tower, or into granules by spraying and tumbling in a rotating drum. The prills or granules may be further dried, cooled, and then coated to prevent caking. These prills or granules are the typical AN products in commerce. The processes involved are simple in principle, but certainly not easy. The Haber process combines nitrogen and hydrogen to produce ammonia, part of which can be oxidised to nitric acid and combined with the remaining ammonia to produce the nitrate. Another production method is used in the so-called Odda process. |
Oxygen donor | ||
Ammonium Perchlorate NH4NO4 A primary oxidizer in many star compositions and other effects. Very impressive colour compositions can be made with it, but their burn rate is often too low for use in star compositions. For lance work and torches slow burning is an advantage and it is therefore commonly used in these items. Produces intense blues and reds when used with copper salts and strontium salts, however mixing with chlorates can create an unstable composition. Ammonium perchlorate is also used in composite rocket propellants, including the propellants used in the solid propellant boosters used for the space shuttle. The decomposition products of ammonium perchlorate are all gasses that are very beneficial for rocket propellants It crystallizes in colorless rhombohedra with a relative density of 1.95. It is the least soluble of all ammonium salts with 20 g in 100 g water at 0 °C, due to the large size of the anion. Like all ammonium salts, it decomposes before fusion. Mild heating results in chlorine, nitrogen, oxygen and water, while strong heating may lead to explosions. |
Ammonium perchlorate can detonate by itself, although it is not very sensitive. Larger amounts and mixtures of ammonium perchlorate with metal powders or organic substances are more likely to detonate. This salt generates toxic gas and extremely high temperature elevation following its decomposition. | Ammonium perchlorate is usually bought from chemical suppliers or from dedicated pyro suppliers. Fine ammonium perchlorate powder is a regulated substance in most countries and cannot easily be bought or transported. It is produced by reaction between ammonia and perchloric acid, or by double decomposition between an ammonium salt and sodium perchlorate. Since it is such a useful chemical in pyrotechnics it can be worth the time and effort to try to prepare it at home. This can be done by first making sodium perchlorate followed by double decomposition with ammonium chloride (other ammonium compounds can be used). The preparation of sodium perchlorate is most easily accomplished by electrolysis. | Oxygen donor | ||
Anthracene C14H10 Used in combination with potassium perchlorate to produce black smokes. |
Physical State; Appearance WHITE CRYSTALS OR FLAKES. Physical dangers Dust explosion possible if in powder or granular form, mixed with air. Chemical dangers The substance decomposes on heating, under influence of strong oxidants producing acrid, toxic fume, causing fire and explosion hazard. Occupational exposure limits TLV not established. Routes of exposure The substance can be absorbed into the body by inhalation. Inhalation risk Evaporation at 20°C is negligible; a harmful concentration of airborne particles can, however, be reached quickly. Effects of short-term exposure The substance slightly irritates the skin and the respiratory tract. Effects of long-term or repeated exposure Repeated or prolonged contact with skin may cause dermatitis under the influence of UV light. | Smoke Ingredient | |||
Antimony Sb The metal is commonly used in the trade as 200-300 mesh powder. It is mainly used with potassium nitrate and sulphur, to produce white fires. It is also responsible in part for the glitter effect seen in some fireworks |
Antimony and many of its compounds are poisonous. Clinically, antimony poisoning is very similar to arsenic poisoning. In small doses, antimony causes headache, dizziness, and depression. Such small doses have in the past been reported in some acidic fruit drinks. The acidic nature of the drink is sufficient to dissolve small amounts of antimony oxide contained in the packaging of the drink; modern manufacturing methods prevent this occurrence. Larger doses cause violent and frequent vomiting, and will lead to death in a few days | Fuel | White, Glitter | ||
Antimony trisulfide SB2S3 Antimony trisulfide is a fuel which is sometimes used in glitter compositions, fountain compositions and flash powder. For the latter purpose however it is used less and less as it is very poisonous and can usually be replaced by sulphur or completely omitted. Used by some who believe it will add a little extra crack to their flash powder reports. Flash compositions containing antimony trisulfide are very sensitive to friction, shock, and static electricity. Handling is a problem because of toxicity and messiness. |
Antimony trisulfide should never be used in any mixture containing chlorates or spontaneous ignition may occur. Mixtures with antimony trisulfide and perchlorates are very sensitive to friction and shock and extra caution should be exercised when handling these mixtures. These mixtures are best avoided at all. Wear proper protective clothing including a dust mask, when working with compositions containing antimony trisulfide as it is very poisonous. | Antimony trisulfide is sometimes sold as a pigment in (art) paint stores, but is not used very commonly these days due to it's toxicity. It can be made at home by fusing a stoichiometric mixture of antimony metal and sulphur. This is a very dangerous operation since extremely toxic fumes will form and it should only be performed with proper safety precautions taken. | Fuel | Glitter | |
Barium Carbonate BaCO3 Barium carbonate is used both in white and fair green color compositions. When chlorine donors are present in a composition a green color will result from the formation of BaCl+ in the flame. When used with Potassium perchlorate it produces a green color of better quality than Barium Nitrate. Without chlorine donors BaO will be formed which emits white light. Barium carbonate is convenient to use in chlorate based color compositions since it will reduce acidity by neutralize residual acid which reduces the risk of spontaneous ignition. Barium carbonate insoluble in water soluble in acid |
Most barium compounds are very poisonous, especially the more soluble barium compounds such as the chlorate and nitrate. A dust mask should be worn at all times when working with barium carbonate. Unlike its soluble cousins which can be easily washed from the hands with lots of water the carbonate is not so easily removed and care to remove the powder from under finger nails is important before eating. Barium carbonate is rat poison and works by interfering with the sodium-potassium pump and causing a paralysis of the muscles, including the heart muscles and respiratory muscles, causing death. | Barium carbonate is cheaply available in kilogram quantities from ceramic supply shops. However, this material is often contaminated with small amounts of barium sulfide that are left over from the production process. Therefore, ceramics grade barium carbonate should never be used in mixtures incompatible with sulfides such as chlorate based mixtures. Barium carbonate is not easily made at home | Color agent, Neutralizer | Green | 590nm |
Barium Chlorate Ba(ClO3)2·H2O Was once used to create greens, but must be used only with compounds that will reduce sensitivity to shock and friction. Barium chlorate is used as an oxidiser in green color compositions. Fierce burning and high color purity compositions can be made with it. Used when deep green colours are needed. It is one of the more sensitive chemicals which are still used, best to avoid if possible, but if used it should be in combination with chemicals which will reduce its sensitivity. |
Barium chlorate is poisonous and a dust mask should be worn at all times when handling it. Barium chlorate should never be mixed with sulfur or sulfides or allowed to come in contact with mixtures containing sulfur or sulfides since this could result in spontaneous ignition. Sulfur reacts with water and air to form small amounts of sulfuric acid. Sulfuric acid and chlorates react producing ClO2, an explosive gas that will ignite many organic materials on contact. Mixtures made with barium chlorate are often especially sensitive to friction and shock (even more so than potassium chlorate based mixtures) and should be handled with extra care | Barium chlorate is usually purchased from chemical suppliers or from dedicated pyro suppliers. It can be made at home from sodium chlorate and barium chloride by double decomposition however purifying the product by recrystallising can be a lot of work because all traces of the sodium must be removed so as to not interfere with pure green colors. Barium chlorate can also be prepared from barium chloride by electrolysis in a process analogous to that used for preparing sodium chlorate. | Color Agent, Oxygen donor | Green, silver | 590nm |
Barium nitrate Ba(NO3)2 Not very strong green effect. Used with aluminum powder to produce silver effects. Below 1000C aluminum burns silvery-gold, characteristic of aluminum-gunpowder compositions. Above 1000C it burns silver, and may be achieved using barium nitrate. Boric acid should always be used in compositions containing barium nitrate and aluminum. Barium nitrate is used as an oxidizer in both white and green color compositions. When chlorine donors are present in a composition a green color will result from the formation of BaCl+ in the flame. Without chlorine donors BaO will be formed which emits bright white light. Barium nitrate is seldom used as the sole oxidizer in green color compositions. It is usually combined with perchlorates to improve the color and increase the burning rate. A poor green effect as a coloring agent by itself. Boric acid often used with compositions with Al. Sometimes used in flash compositions. |
Barium nitrate is poisonous and a dust mask should be worn at all times when handling it. Mixtures of metal powders and barium nitrate sometimes heat up spontaneously and may ignite, especially when moist. This can usually be prevented by the addition of small amounts of boric acid (1 to 2%). It is advisable to avoid using water to bind such compositions. Red gum or shellac with alcohol or nitrocellulose lacquer are preferred binder and solvents Causes irritation to the respiratory tract. Symptoms may include coughing, shortness of breath. Systemic poisoning may occur with symptoms similar to those of ingestion. If ingested it may cause tightness of the muscles of the face and neck, vomiting, diarrhea, abdominal pain, muscular tremors, anxiety, weakness, labored breathing, cardiac irregularity, convulsions, and death from cardiac and respiratory failure. Estimated lethal dose lies between 1 to 15 grams. Death may occur within hours or up to a few days. May cause kidney damage. Causes irritation to skin. Symptoms include redness, itching, and pain. If it comes into contact with eyes it causes irritation, redness, and pain Inhalation: Causes irritation to the respiratory tract. Symptoms may include coughing, shortness of breath. Systemic poisoning may occur with symptoms similar to those of ingestion. Ingestion: Toxic! May cause tightness of the muscles of the face and neck, vomiting, diarrhea, abdominal pain, muscular tremors, anxiety, weakness, labored breathing, cardiac irregularity, convulsions, and death from cardiac and respiratory failure. Estimated lethal dose lies between 1 to 15 grams. Death may occur within hours or up to a few days. May cause kidney damage. Skin Contact: Causes irritation to skin. Symptoms include redness, itching, and pain. Eye Contact: Causes irritation, redness, and pain. |
Barium nitrate may be prepared from nitric acid or ammonium nitrate and barium carbonate, which is available from ceramic supply stores. It can also be made from sodium nitrate and barium chloride by double decomposition and decentralizing for purity. It should be done outside with an electric hotplate and stainless steel ware. Garden hose at the ready and nothing left outside for the kids too handle. Wash any spills into the ground with the hose until below the surface. Spread some ammonium sulphate fertilizer over and water some more. This will convert soluble barium salts to insoluble barium sulphate, which is harmless. Neutralize all waste solution with enough ammonium sulphate until white clouds of powder is no longer seen in the clear liquid then it is safe to dump onto ground | Color agent, Enhancer, and Oxygen donor | Green, silver | 590nm |
Barium Oxalate BaC2O4·H2O Rarely used in fireworks. Sometimes used, signaling applications with magnesium. but sometimes used in signaling applications |
A mild skin irritant, the substance is considered toxic when ingested, causing nausea, vomiting and renal failure. | Color Agent, Oxygen donor | Green, silver | 590nm | |
Barium sulfate BaSO4 An excellent green color agent and oxidizer for strobe compositions. Sometimes used for glitter compositions as a delay. Barium sulfate is used as a high temperature oxidizer in certain pyrotechnic formulas, as it produces a green colored light while it burns. Barium nitrate is more common in green pyrotechnic formulas, as it is a more amiable oxidizer while still producing green colored light. |
Unlike many other barium compounds, barium sulfate is not very poisonous due to its low solubility in water. | Barium sulfate may be precipitated from a solution of a soluble barium salt, such as barium nitrate or chloride, and a sulfate. Magnesium and potassium sulfate are both cheaply available as fertilizer and are convenient to use. The precipitated barium sulfate is a very fine powder which may be rinsed by repeated washings with hot water, settling and decanting. A final washing in the filter with acetone or ethanol will allow it to dry quickly. Do not use sulfuric acid to precipitate barium sulfate as this may result in the inclusion of acid droplets in the precipitated particles which can lead to spontaneous ignition of some mixtures. | Color Agent, Oxygen donor | ||
Black powder The basis for all fireworks. Potassium nitrate, charcoal and sulfur (75:15:10) |
Propellant, explosive, primer | ||||
Boric acid H3BO3 Boric acid is a white powder which is used as an additive to compositions containing aluminum or magnesium and a nitrate. The metal powder can reduce the nitrate to an amide, which will react with the metal powder in a very exothermic reaction that can lead to spontaneous ignition of the composition. This process is often accompanied by a smell of ammonia and is most likely to occur with wet compositions. Addition of a few percent boric acid can often prevent this reaction from taking place since it neutralizes the very basic amides forming ammonia and a borate. It is also advisable to avoid using a water-soluble binder for these compositions. Using red gum or shellac with alcohol or nitrocellulose lacquer is safer. |
Boric acid is not particularly toxic or dangerous. | Boric acid is cheaply and in kilogram quantities available from ceramic supply shops. It is also sold in many drug stores at a somewhat higher price, but since only small quantities are needed the price is not really important. It is also sold in Home centers as an effective insecticide for roach's (it may list the contents as orthoboric acid). | Neutralizer | ||
Calcium carbonate CaCO3 Used as a neutralizer in mixtures that are sensitive to both acids and bases, for example chlorate/aluminum flash powder. |
Not hazardous. | The vast majority of calcium carbonate used in industry is extracted by mining or quarrying. Pure calcium carbonate (e.g. for food or pharmaceutical use), can be produced from a pure quarried source (usually marble) or it can be prepared by passing carbon dioxide into a solution of calcium hydroxide: the calcium carbonate precipitates out, and this grade of product is referred to as a precipitate (abbreviated to PCC). | Neutralizer | ||
Calcium oxalate CaC2O4 Used to add depth to colors produced by other metal salts. Gives improved color saturation to mixtures of Sodium Nitrate and Magnesium. Calcium Oxalate can also be used in some pyrotechnic formulations to intensify a red color. In compositions with metal salts color Agent. |
Even small doses of oxalate toxin is enough to cause intense sensations of burning in the mouth and throat, swelling, and choking. In larger doses, however, Oxalate causes severe digestive upset, breathing difficulties and - if enough is consumed - convulsions, coma and death. Recovery from severe oxalate poisoning is possible, but permanent liver and kidney damage may have occurred. Avoid contact with skin and avoid ingestion. Use dust respirator when handling. Since no exposure limit has been established for Calcium Oxalate by OSHA & ACGIH | Color enhancer | |||
Calcium silicide CaSi2 Used in Smoke Composition . |
Reacts with water to form Silane and Calcium Hydroxide. Irritating to skin, eyes and mucous membranes. Consider toxic all routes. The chemical, physical and toxicological properties have not been fully investigated. LD/TD no data. OSHA PEL/ACGIH TLV not established. No evidence of carcinogenicity. Ingestion may cause burning sensation in mouth, throat and stomach. |
Smokes | |||
Calcium sulfate CaCO4 (Plaster of Paris or Gypsum) Calcium sulphate can be used as a high temperature oxidizer in orange color compositions. Excellent reddish-orange color agent in strobe compositions. The trihydrate is commonly known as plaster of paris. The dihydrate occurs as a mineral known as gypsum. Natural, unrefined calcium sulfate is a translucent, crystalline white rock. After being heated and crushed into a powder, it is often used as the coagulant in soy processing, such as making tofu. Its most common use is in blackboard chalk. A good high temperature oxidizer and occasionally used in strobe formulas. |
Calcium sulphate is not particularly toxic or dangerous. | Plaster can be used as is in strobe compositions, but is better to remove the water which is easily accomplished by heating. Plaster of Paris can be bought at hardware's etc. | Color Agent, Oxygen donor | Orange, red | 670nm |
Carbon C (Lampblack) Very dirty to work with but makes wonderful stars for willow and spider style aerial shells. Used in fireworks to produce very long lasting, finely dispersed dim gold sparks; in rockets, used to opacify rocket fuel grains. The opacifier accelerates the rate of surface burning and prevents infrared energy from penetrating the propellant grain and causing motor pre-ignition. |
Avoid inhalation and ingestion. Avoid storing in excessive temperatures. A dust respirator should be worn. | Lampblack is easily produced experimentally by passing some noncombustible surface, such as a tin can lid or glass, closely through a candle flame. Lampblack produced in this way is among the darkest and least reflective substances known[. | Fuel | Orange, gold | 670nm |
Carbon C (Charcoal) Charcoal finds widespread use in pyrotechnics. Many types of charcoal exist, each with its own properties. The primary fuel source in black powder and various formulas. There are a variety of types of charcoal dependent on the source of the wood or fiber. The type of charcoal can have a dramatic impact on the quality and performance of the black powder. It is a complex organic substance containing moisture, ash, carbon, hydrogen, oxygen and a variety of volatiles. All of these elements have a vital use in fireworks. Charcoal made from willow or grapevine is considered great for black powder, while paulownia and pine charcoal are commonly used for spark effects. The particle size and the process used to make the charcoal also play an important role in the quality of the charcoal for a specific purpose. Very fine charcoal floats in air and is therefore sometimes referred to as 'airfloat'. |
Fine charcoal dust is easily breathed in, and a dust mask should be worn when working with it. Freshly prepared charcoal can be pyrophoric even when not powdered and it must be allowed to stand for a day at least before it is used to prepare compositions with. | Barbeque briquettes are mixed with clay and are not suitable for making black powder. Charcoal can be purchased from supermarkets, BBQ supply stores and directly from online pyrotechnic chemical suppliers Charcoal can easily be prepared at home and a basic tutorial is outlined below. |
Fuel | Orange, gold | 670nm |
Castor oil Used to coat sensitive metals to protect them during processing, especially magnesium.Used in composite formulations using Polybutadienes and polyesters. Ninety percent of fatty acids in castor oil are ricinoleic acid. |
Stabilizer | ||||
Chlorine Cl Provided in Saran ™ and, Parlon ™ or PVC. |
Color intensifier | ||||
Copper acetoarsenite Cu3As2O3Cu(C2H3O2) 2 (Paris Green) Commonly called Paris Green, this chemical is toxic but used to produce some of the best blue colors in combination with potassium perchlorate. Rarely used because of toxicity but makes the one of the best blues. |
Copper acetoarsenite is very poisonous and should only be handled wearing a dust mask. Smoke from compositions containing this compound should not be inhaled. It is best to avoid the use of this compound altogether as several safer alternatives have become available in the past decades. | Copper acetoarsenite was used in the past as a pigment known as emerald green, kings green or vienna green. Nowadays it is no longer used and it is very hard to find a paint supplier that still has it. It can be prepared at home but extreme caution must be exercised since arsenic compounds are very poisonous. The following preparation originates from Shimizu: "300 g of copper sulphate is dissolved in 1000 ml water, to which 250 g of glacial acetic acid is added; This solution is named 'A'. Then 200 g of sodium carbonate and 200 g of ersenious acid are added to 1000 ml water and boiled to form a solution, this is named 'B'. B is added little by little to A with constant stirring. Carbon dioxide gas is generated with active bubbling. When all the solution B has been added, it is boiled for about 30 minutes, when copper acetoarsenite appears gradually as green particles in the solution. The mother liquor is removed by vacuum filtration, and then green substance, copper acetoarsenite, is washed with water untill the sulphate ion disappears; it is then dried. The yield is about 180 g." Dissolve in a small quantity of hot water, 6 parts of sulphate of copper; in another part, boil 6 parts of oxide of arsenic with 8 parts of potash, until it throws out no more carbonic acid; mix by degrees this hot solution with the first, agitating continually until the effervescence has entirely ceased; these then form a precipitate of a dirty greenish yellow, very abundant; add to it about 3 parts of acetic acid, or such a quantity that there may be a slight excess perceptible to the smell after the mixture; by degrees the precipitate diminishes the bulk, and in a few hours there deposes spontaneously at the bottom of the liquor entirely discolored, a powder of a contexture slightly crystalline, and of a very beautiful green; afterwards the floating liquor is separated. |
Color agent | Blue | 500-535 |
Copper carbonate CuCO3 This is the best copper compound for use with ammonium perchlorate for production of blue colors. In addition to being a coloring agent, the carbonate can neutralize acids formed during storage, thus helping to stabilize KCIO3 mixtures. However, since copper is known to sensitize chlorates. this benefit is minimal at best. Frequently used with ammonium perchlorate. |
Avoid inhalation and ingestion. Wash hands thoroughly after handling. Use dust respirator when handling. OSHA PEL and ACGIH TLV-TWA have been established for this compound at 1mg/m3. This product may be subject to SARA 313 reporting requirements (Cu 57.47%). | Color agent | Blue | ||
Copper chloride CuCl The richest blue flame agent of all and flexible with a number of oxidizers. Good hygroscopic properties. Copper(I) chloride (quite commonly called cuprous chloride), is the lower chloride of copper, with the formula CuCl. It occurs naturally as the mineral nantokite. It is a white solid which is almost insoluble in water, and which tends to oxidize in air to green CuCl2. |
Copper salts do have some toxicity and should be handled with care; wear gloves and goggles. Avoid bringing CuCl into contact with alkynes. | Copper(I) chloride may be prepared by the reduction of copper(II) salts such as CuSO4 using sulfur dioxide or copper metal. SO2 may be prepared in situ from sodium bisulfite (NaHSO3) or sodium metabisulfite (Na2S2O5) and acid. The reduction is carried out in hydrochloric acid, and the resulting CuCl2- complex is diluted to precipitate white CuCl | Color agent | Blue | |
Copper metal Cu Rarely used since other compounds are easier to work with and more effective. Copper powder is a rusty orange colored fine powder which was used not so long ago in the manufacture of stars to achieve a green and blue colors. Atomized powder is particularly well suited to the strobe applications. It is no longer used today as it is replaced with chemicals like barium and copper oxides. |
The metal, when powdered, is a fire hazard. At concentrations higher than 1 mg/L, copper can stain clothes and items washed in water. | Copper powder can be purchased through craft or ceramic stores. | Color agent | Blue, Green | |
Copper(II) oxide CuO Excellent blues in composite stars. Copper oxide is a black powder employed in blue color compositions in combination with chlorine donors. |
Copper(II)oxide is poisonous and should be handled wearing a dust mask. | Copper(II)oxide is usually available from ceramic supply stores. It is also easily prepared at home as follows: Add a solution of sodium or potassium hydroxide to a solution of a soluble copper(II) compound (copper sulfate for example). This will yield a blue gel-like precipitate of copper(II)hydroxide. Then bring to solution to a boil. The precipitate will turn black and powdery. Boil for a minute or two to complete the reaction and allow the black copper(II)oxide precipitate to settle. Then decant the liquid. Add some boiling hot water to the precipite, stir and allow to settle again. Then decant and repeat 5 more times. This will remove all soluble impurities from the copper(II)oxide. Then the precipitate is filtered and allowed to dry. | Oxygen donor, color agent | Blue | |
Copper oxychloride Cu2O Occasionally used in cheap blue compositions. Not used much in modern fireworks because of the need for mercury chloride to bring out the color. |
Avoid breathing dust. Avoid getting in eyes or on skin. Wash thoroughly after handling. Store in a dry place away from direct sunlight, heat and incompatible materials. Reseal containers immediately after use. Store away from food and beverages. | Oxygen donor, color agent | Blue | ||
| Copper salts (except copper chlorate) | Color agent | Blue | |||
Cryolite Na3AlF6 (Greenland spar) Also known as Greenland spar, this is an insoluble sodium salt. Sodium salts are used to produce yellow colors, but as sodium salts generally absorb water this tends to be a problem. By using cryolite this problem is surmounted. A sodium salt that does not absorb water, making it ideal for use in fireworks compositions. |
Avoid breathing dust. Avoid getting in eyes or on skin. Wash thoroughly after handling. Store in a dry place away from direct sunlight, heat and incompatible materials. Reseal containers immediately after use. Store away from food and beverages. Overexposure may cause fluorosis, which is a condition affecting the bones and teeth. See MSDS for additional information. | Color agent | Yellow | ||
Dextrine (C6H10O5)n The most regularly used binder for compositions. Cheap, easy to use and work with, water soluble and holds most formulas together well after drying. In pyrotechnics they are added to colored fire formulas, allowing them to solidify as pellets or "stars." |
Dextrin is not particularly toxic or dangerous. | Dextrin is easily prepared from starch. Potato and cornstarch will both work fine. The starch is spread out on a sheet in a layer about 1 cm thick and placed in the oven. The oven is then heated to 220°C for several hours. The dextrin will turn slightly yellowish brown. One way to check if all the starch has been converted is to dissolve a small sample in boiling hot water and add a drop of KI3 solution. A blue color indicates presence of starch, which means the conversion hasn't completed yet. KI3 solution is conveniently prepared by dissolving a crystal of elemental iodine in a potassium iodide solution. | Fuel, binder | ||
Gallic acid (3,4,5-trihydroxybenzoic acid) C7H6O55·H2O This is used in some formulas for whistling fireworks. Whistle mixes containing gallic acid are generally the most sensitive of the whistling fireworks, with high sensitivity to both friction and impact when used with chlorates, but cannot be used with perchlorates either. There are safer alternatives for whistle compositions. Sensitive to impact and friction if combined with potassium chlorate and even potassium perchlorate. Not commonly used. |
Fuel | Whistles | |||
Gum resins (accroid resin, shellac, gum copal and red gum) Less commonly used than Dextrine, but works well for specific mixtures. |
Fuel, binders | ||||
Hexachlorobenzene C6Cl6 Until it stopped being manufactured in the US due to its toxicity in the 1970’s, hexachlorobenzene was the most widely used chlorine donor in fireworks. Used as a chlorine donor in colored compositions that require one. Rarely used now, with PVC, Saran and Parlon being preferred, as they are more efficient. |
Hexachlorobenzene is an animal carcinogen and is considered to be a probable human carcinogen. American Pellagra was a disease affecting 250,000 people between 1900-1950 caused by hexachlorobenzene residue from new bleaching and degermination procedures for corn and wheat. Hexachlorobenzene was banned from use in the United States in 1966. | Color Enhancer | |||
Hexachloroethane C2Cl6 Hi-temp oxidizer in smoke compositions.More often found in military devices. More often found in military devices. |
Moderately irritating to skin and mucous membranes. Mechanical ventilation required as required as required by OSHA and ACGIH air level consideration. Avoid inhalation and ingestion. | Smoke | |||
| Hexamethylenenetetramine (hexamine) (CH2)6N4 | Mechanical exhaust required. Avoid prolonged exposure. Keep containers sealed. Store in cool, dry place. Keep away from heat, open flame. Suspected carcinogen. Avoid inhalation and ingestion. Wash thoroughly after handling. | Oxygen enhancer | Yellow | ||
Iron Fe The metal filings are one of the oldest sources for "color" in fireworks. Usually linseed oil is mixed with it to coat it and protect it because the filings rust so quickly.Iron powder is used for spark effects, mainly in fountains and sparklers. It produces golden yellow branching sparks. Not every iron alloy will work equally well. Iron alloys with a high carbon content generally work best. Stainless steel will produce hardly any sparks. |
Iron needs to be protected before use in pyrotechnic compositions. Otherwise it will corrode and render the composition useless or even dangerous. Iron containing compositions are generally best kept dry and not bound with water soluble binders. Iron can be coated with linseed or tung oil. The latter was used in ancient China (and may still be used today). Linseed is very convenient to use and easy to obtain. Black powder-like compositions (ie Charcoal/sulfur/saltpeter based) with added metal, such as they are often used in fountains, are more sensitive than the composition without added metal. Extra caution, especially when pressing or ramming, should be exercised. | Iron turnings can often be had for free from places were iron is used for construction. Drilling, sawing etc produces a powder with wide range of particles. This powder is treated with mineral oil to remove oil and grease, sieved, and then coated with linseed oil. | Fuel | Silver | |
Lactose C12H22O11·H2O (Milk sugar) Lactose is a binder in pyrotechnic compositions. A cheap, easy fuel for smoke devices. Used in smoke formulations; as a low reactivity (accessory) fuel, often in blue star formulations. |
Fuel | Smoke | |||
Linseed oil Used to brighten some effects and colors. Difficult to work with because of sensitivity and because it burns at such a high temperature, washes out any other colors |
Stabilizer | ||||
Lithium carbonate Li2CO3 Poor color agent, used in some SPFX but mostly replaced by strontium salts. |
Color agent | Red | 650nm | ||
Magnalium (magnesium-aluminum alloy) MgAl Magnalium is a very brittle alloy of magnesium and aluminum. Some common uses are in for spark effects, in strobing compositions and in crackling stars. A more stable and less expensive alloy to replace magnesium in some compositions. Not as reactive as magnesium, and not as hard to ignite as aluminum. Easier to work with, safer and less expensive. |
Magnalium dust is harmful and a dust mask should be worn when handling fine dust. Mixtures containing nitrates and mangalium sometimes heat up and may ignite spontaneously, especially when moist. This can usually be prevented by treating the magnalium with potassium dichromate. This is done by boiling the magnalium in a 5% potassium dichromate solution. Adding fine potassium dichromate powder to such compositions may also help. | Magnalium can be made at home. Plan well and prepare yourself for working with molten metals that may ignite if you plan to make it at home. If the metal ignites expect it to burn very brightly and hot. Explosions are not common but may occur if the hot melt is allowed to contact water or oxidisers. Do it outside and away from anything flammable. If it ignites don't try to extinguish it but get away from the burning mass and let it burn out and cool before approaching it. Don't look directly into the burning metal as it may damage your eyes. Start by melting aluminum in a stainless steel container. The molten metal should be covered with a blanket of inert gas. In this case neither nitrogen nor carbon dioxide will function as an inert gas. It is best to get a cylinder of argon gas at a welding supply store. Using an electric furnace for the melting is very convenient and allows good control over the temperature. To the molten aluminum, magnesium is added in solid form. The melt should be stirred from time to time. When all the magnesium has melted, the melt is allowed to solidify. It is then easily crushed up in smaller chunks with an heavy hammer. These chunks are crushed further and sieved. It can also be ball milled into a fine powder using steel media but this can be dangerous since the metal powder can become pyrophoric. | Fuel | Silver, white | |
Magnesium Mg Magnesium powder is used in a wide variety of compositions, both for spark effects and 'normal' fuel purposes. Relatively coarse magnesium is used for spark effects. In flares and some bright colored star compositions it functions as a normal fuel. It is superior to aluminum in color compositions since MgCl2 and MgO are more easily vaporized than the corresponding aluminum compounds. This reduces the amount of black-body radiation and improves the color purity. Used to brighten some effects and colors. Difficult to work with because of sensitivity and because it burns at such a high temperature, washes out any other colors |
Magnesium dust is harmful and a dust mask should be worn when handling fine dust. Mixtures containing nitrates and magnesium sometimes heat up and may ignite spontaneously, especially when moist. This can usually be prevented by treating the magnesium with potassium dichromate. This is done by boiling the magnalium in a 5% potassium dichromate solution. The magnesium will turn brown when this is done. Adding fine potassium dichromate powder to such compositions may also help. | Magnesite, or magnesium carbonate (MgCO3), has a theoretical magnesium content of 47.6 percent. Dolomite is a calcium carbonate-magnesium carbonate mineral (CaCO3CMgCO3) that has a theoretical magnesium content of 22 percent. Brucite, magnesium hydroxide [Mg(OH)2], contains up to 69 percent magnesium, and olivine (Mg2Fe2SiO4) contains up to 19 percent magnesium. Of these minerals, magnesite and dolomite are the largest sources of magnesium and magnesium compounds. Seawater, brines, and bitterns represent vast sources of magnesium and magnesium compounds. In the United States, about 60 percent of the magnesium compounds produced annually is recovered from seawater and brines, and more than one-half of the magnesium metal production capacity uses seawater or brines as a raw material. Various magnesia products are made by calcining magnesium carbonate or magnesium hydroxide at different temperatures. Caustic-calcined magnesia, which is readily reactive with water, is calcined at temperatures up to 890E C. Dead-burned magnesia, also called refractory or sintered magnesia, is calcined at temperatures up to 1,450E C and is unreactive with water. Fused magnesia is produced at temperatures greater than 3,000E C. Magnesia produced from magnesite is generally called natural magnesia, and magnesia produced from seawater or brines is called synthetic magnesia. | Fuel | White | |
Magnesium carbonate MgCO3 Helps potassium chlorate or perchlorate flow free. Also used as neutralizer and in some smoke formulations. |
Adequate ventilation required. Keep containers sealed. Store in cool, dry place. | Neutralizer | |||
Magnesium sulfate Magnesium sulfate or Magnesium sulfate heptahydrate or Epsom salt is a chemical compound containing magnesium, with the formula MgSO4·7H2O. Magnesium sulfate without water of crystallization MgSO4 is available as a far less common chemical and drying agent, but typically "magnesium sulfate" refers to the hydrate, and Epsom salt always refers to the hydrate. |
Oxygen donor | ||||
Nitrocellulose-based lacquers Use primarily in safety fuses to provide waterproofing. |
Binder | ||||
Parlon ™ (Chlorinated isoprene rubber) Parlon is a chlorine donor, and a key ingredient in many colored stars. It is a chlorinated isoprene rubber, chlorine content 66%. It interferes with burning less than PVC or saran, and can be used as a binder. It is soluble in methyl ethyl ketone (MEK) and partially in acetone. Compositions made with parlon and acetone or MEK are nearly waterproof. Parlon is a acetone-soluble polymer that is used as a chlorine donor and binder. It is a good example of one of the new chemicals that has become available in the past few decades for use in compositions. |
Parlon is not particularly dangerous. | Parlon seems to be available from dedicated pyro suppliers only. | Color enhancer, Binder | ||
Petroleum jelly (Vaseline) Occasionally used to protect metal powders e.g. iron by coating them with a thin film of petroleum jelly. |
Stabilizer | ||||
Phosphorus P In fireworks, red phosphorous is used only in toy caps and party poppers. Phosphorous makes compositions very sensitive to shock and friction and combining it with many pyrotechnic formulas can result in an explosion on contact. This is why it is used to tip strike anywhere matches and signal devices. Yellow phosphorous is used only in some military applications. Phosphorous is also toxic to breathe or handle and requires special facilities for storage and manufacturing and personal protective equipment to handle. Phosphorus is rarely used in pyrotechnics today, except for a few specialized applications. It was used commonly many years ago, but as the hazards associated with its use became known it dropped out of use. Phosphorus comes in several forms, of which the red and the white/yellow varieties were used. Red phosphorus (used in the strikers on the side of matchboxes) is the more stable form, while white phosphorus (used by the military in incendiary devices) ignites spontaneously in air, and must therefore be stored under water or otherwise protected from the atmosphere. Both forms are toxic. |
The element is obtained industrially by the heating phosphate rock in sand (SiO2) and coke (carbon) in an electric furnace of 1450oC. The phosphorus produced is P4 and collected under water as white phosphorus. The element is obtained industrially by the heating phosphate rock in sand (SiO2) and coke (carbon) in an electric furnace of 1450oC. The phosphorus produced is P4 and collected under water as white phosphorus. | Fuel | |||
Phosphorus (red) P Phosphorus is rarely used in pyrotechnics today, except for a few specialized applications. It was used commonly many years ago, but as the hazards associated with its use became known it dropped out of use. |
Fuel | ||||
Polyester A more efficient coating of metal powders to protect them than the oils. |
Stabilizer | ||||
Polyvinyl chloride (PVC)(CH2=CHCl)n (PVC) Like parlon and saran, PVC is a polymeric chlorine donor and fuel. It can be used in the form of a fine powder or as a solution in tetrahydrofuran (THF). It is sometimes used as a binder, but it is very brittle. Small amounts of plasticiser (dioctyl phtalate is common) may be added to improve the mechanical properties. PVC is a commonly used chlorine donor. It is not as good as Parlon for this purpose, but is cheaper and more readily available. PVC is soluble in Soluble in MEK, Methylene chloride, cyclohexane or tetrahydrofuran (THF) but almost all other solvents are useless. Methyl ethyl ketone (MEK) will plasticise PVC to some extent, however. A chlorine donor sometimes used in green star formulations |
PVC itself is not particularly dangerous or toxic. Dioctyl phtalate is a suspected carcinogen however and THF is a very flamable and volatile liquid. | As an alternative to the PVC powder available from chemical suppliers and dedicated pyro suppliers, PVC glue may also be used. It is usually sold in hardware stores and comes in two varieties: gelling or gap-filling and normal. Both are essentially a concentrated solution of PVC. I have no experience with the gelling variety, but the normal variety can successfully be used in compositions. The gelling variety may be better suited for pyro purposes since it seems it contains more PVC. Another possibility is to use 'Sculpy' or 'Fimo' clay. These modeling clays consist of PVC with a large amount of plasticiser. The plastics may affect the color of a composition negatively, but reasonable results can still be obtained with it. It can simply be kneaded into a composition with some effort. This type of clay is usually hardened by heating it in an oven, but do not be tempted to do this with pyrotechnic mixtures as they may ignite. | Color enhancer | ||
Potassium benzoate C6H5CO2K One of the more common whistle ingredients. Used in whistling fireworks, in combination with potassium perchlorate. It must be very dry for this purpose, and should be less than 120 mesh.. Requires some special conditions for safe mixing and pressing. |
Fuel | Whistle | |||
Potassium chlorate KClO3 An important oxygen donor, however in the presence of sulfur, ammonium salts, and phosphorus or any acidity, it creates an extremely high shock and friction risk. Some fireworks makers use it to improve the performance of their products, especially fuses and quickmatch and some star formulas. Others use it because it costs less and is more easily obtained even though it has some treacherous properties and other oxidisers would sometimes be safer to use.Potassium chlorate was a very common oxidiser in pyrotechnics. Banned in English fireworks since 1875. Part of the reason of its popularity in commercial pyrotechnics is that it is cheap and easily available. The large scale production of this compound made the first quality colored fireworks possible, about a century ago. |
Potassium chlorate is toxic, and breathing protection should be worn when handling fine powder. Compositions made with potassium chlorate tend to be more sensitive than those based on nitrates and perchlorates and should therefore be handled accordingly. Potassium chlorate, or any chlorate for that matter, should never be used in combination with sulfur and sulfides. Mixtures containing both are very sensitive and may spontaneously ignite. In general, when using chlorates great care should be taken to avoid contamination of other compositions or tools. Also read the general safety page for more information on this problem. http://www.hummelcroton.com/msds/kclo3_m.html |
Oxygen donor | |||
Potassium dichromate K2Cr2O7 Generally found with potassium perchlorate compositions. |
Oxygen donor | ||||
Potassium hydrogen phthalate
|
Fuel | Whistle | |||
Potassium nitrate KNO3 (Saltpeter) A basic ingredient of gunpowder and a number of other common formulations. |
Oxygen donor | ||||
Potassium or sodium benzoate
|
Fuel | Whistle | |||
Potassium perchlorate KClO4 The primary oxidizer used in most fireworks because of its excellent performance and stability. Potassium perchlorate is a very common oxidizer in pyrotechnics. Composition based on perchlorates tend to be less sensitive than those based on chlorates, and perchlorates can be used with sulfur and sulfides. For these reasons potassium perchlorate is much preferred above chlorates. Drawback is its slightly higher price. This is a shock sensitive compound that is used in some whistle formulas. While safer than gallic acid formulas in this respect, care should be taken to keep it away from other metals such as lead, because some other metallic picrates are extremely sensitive. |
Fire and explosion hazard when in contact with reducing agents (fuels). Hazardous when mixed with organics. Since no exposure limits have been established by OSHA and ACGIH, we recommend that our product be treated as a nuisance dust | Oxygen donor | |||
Potassium picrate C6H2(NO2)3OK Very sensitive to shock and friction, so used very rarely. |
Fuel | Whistle | |||
Potassium sulfate K2SO4 Mostly used in strobe formulas. |
Oxygen donor | ||||
Rosin Colophonium C20O2H29 Colophonium is an alcohol soluble resin which is sometimes used as a binder and as a fuel-binder combination . It is not used very often since it is expensive and doesn't have much adhesion capacity. Mixture of compounds, mainly abietic acid |
Colophonium is not particularly toxic or dangerous | Rosin, formerly called colophony or Greek pitch (Pix græca), is a solid form of resin obtained from pines and some other plants, mostly conifers, produced by heating fresh liquid resin to vaporize the volatile liquid terpene components. It is semi-transparent and varies in color from yellow to black. At room temperature it is brittle, but it melts at stove-top temperatures. It chiefly consists of different resin acids, especially abietic acid. Artist paint stores often sell colophonium. It is also used by violin players, for the treatment of wooden floors and in the paper industry. | |||
Saran ™ The most commonly used chlorine donor. Easy to work with, inexpensive and stable performance. |
Color Enhancer, Binder | ||||
Sodium bicarbonate (sodium hydrogen carbonate) NaHCO3 (Baking soda) A fair to poor yellow coloring agent. Generally a delay agent in some glitter compositions. |
Color agent, Neutralizer | Yellow | |||
Sodium nitrate NaNO3 (Chile saltpeter) sodium nitrate is sold as a food preservative, 95% pure sodium nitrate is also available as a fertilizer. In the Netherlands this fertilizer is sold under the name 'chilisalpeter'. If required, it can be easily purified by recrystallisation. It is a good raw material for manufacture of barium nitrate. |
Oxygen donor | Yellow | |||
Sodium oxalate Na2C2O4 Another fair to poor yellow and delay agent. |
Oxygen donor | Yellow, glitter | |||
Sodium salicylate Very useful whistle fuel. |
Fuel | Whistle | |||
Sodium silicate (Water glass) Only limited uses in some items. |
Neutralizer | ||||
Sodium salts (except sodium ch1orate) Inexpensive but hygroscopic, they are sometimes used in place of potassium salts. |
Color Agent | Orange, yellow | |||
Sodium sulphate Na2SO4 Sometimes used in some yellow strobe compositions.
|
Color agent, Oxygen donor | Yellow | |||
Starch (C6H10O5)n (Rice starch corn starch) Binds some star compositions and reduces burning speeds |
Fuel/Binder | ||||
Stearine (Stearic acid) Lengthens flames and reduces some friction sensitivity in some compositions. Used as a phlegmatizing agent, a low reactivity (accessory) fuel, sometimes in blue star compositions. It can often take the place of Sulfur and Charcoal in fireworks. Stearic acid, also called octadecanoic acid, is used in lubricants, pyrotechnics, soaps, dispersing agents, shoe and metal polishes and accelerator activators. |
Fuel | ||||
Strontium carbonate SrCO3 Produces a good red, sometimes used to slow some mixtures burning. Used often for producing red colours, and as a fire retardant in gunpowder mixtures. An inexpensive colorant in fireworks, Strontium and its salts emit a brilliant red color in flame. Unlike other strontium salts, the carbonate salt is generally prefered because of its cost and the fact that it is not hygroscopic. Its ability to neutralize acid is also very helpful in pyrotechnics. Another similar application is in road flares. Strontium carbonate is a white, odorless, tasteless powder. It's chemical makeup is: C 8.14% O 32.51% Sr 59.35%. Being a carbonate, it is a weak base and therefore is reactive with acids. It is otherwise stable and safe to work with. It is practically insoluble in water (1 part in 100,000). The solubility is increased significantly if the water is saturated with CO2, to 1 part in 1,000. It is soluble in dilute acids. |
Accute Health Effects: Irritating to the skin and eyes on contact. Inhalation will cause irritation to the lungs and mucus membrane. Irritation to the eyes will cause watering and redness. Reddening, scaling, and itching are characteristics of skin inflammation. Follow safe industrial hygiene practices and always wear protective equipment when handling this compound. Chronic Health Effects: This product has no known chronic effects. Repeated or prolong exposure to this compound is not known to aggravate medical conditions. Accute Health Effects: This product is not listed by NTP, IARC or regulated as a Carcinogen by OSHA. | Strontium carbonate is cheaply available in kilogram quantities from ceramic supply shops. However, this material is often contaminated with small amounts of strontium sulfide which are left over from the production process. Therefore, ceramics grade strontium carbonate should never be used in mixtures incompatible with sulfides such as chlorate based mixtures. Strontium carbonate is not easily made at home | Color agent, Retardant | Red | 650nm |
Strontium nitrate Sr(NO3)2 Strontium nitrate is an oxidiser commonly employed in red color compositions in combination with chlorine donors. Used in road flares (fusees) and many other red compositions because of the excellent red, especially in combination with metal fuels |
Strontium Nitrate can affect you when breathed in. Contact can irritate the skin and eyes. Breathing Strontium Nitrate can irritate the nose and throat. Repeated exposure may damage the lungs, heart, liver and kidneys, and affect the nervous system Exposure to very high levels of Strontium Nitrate can cause it to accumulate in the bones and may affect their function. | Strontium nitrate may be prepared from nitric acid or ammonium nitrate and strontium carbonate, which is available from ceramic supply stores. Use an excess of strontium carbonate to ensure complete neutralisation of acid and recrystallise the product from a slightly alkaline solution to prevent the inclusion of acid solvent droplets in the crystals. | Color agent, Oxygen donor | Red | 650nm |
Strontium oxalate SrC2O4·H2O A fair red color but the water content slows it down too much. |
Color agent, Retardant, Neutralizer | Red, glitter | 650nm | ||
| Strontium salts (except strontium chlorate) | Color agent | Red | 650nm | ||
Strontium sulfate SrSO4 A useful ingredient in red strobe mixtures. Strontium sulfate is used as a high-temperature oxidizer in some metal based red color compositions. |
Strontium sulfate is not particularly dangerous or toxic. | Strontium sulfate may be precipitated from a solution of a soluble strontium salt, such as strontium nitrate or chloride, and a sulfate. Magnesium and potassium sulfate are both cheaply available as fertilizer and are convenient to use. The precipitated strontium sulfate is a very fine powder which may be rinsed by repeated washings with hot water, settling and decanting. A final washing in the filter with acetone or ethanol will allow it to dry quickly. Do not use sulfuric acid to precipitate strontium sulfate as this may result in the inclusion of acid droplets in the precipitated particles which can lead to spontaneous ignition of some mixtures. | Color agent, Oxygen donor | Red | 650nm |
| Sucrose C12H22O11 (Beet or cane sugar) | Fuel | Smoke | |||
Sulfur S At room temperature, sulfur is a soft bright yellow solid. Although sulfur is blamed for the smell—, e.g. of rotten eggs— elemental sulfur has only the faintest odor (the odor associated with rotten eggs is actually due to hydrogen sulfide and organic sulfur compounds). It burns with a blue flame that emits sulfur dioxide, notable for its peculiar suffocating odor. Sulfur is insoluble in water but soluble in carbon disulfide and to a lesser extent in other organic solvents such as benzene. Common oxidation states of sulfur include −2, +2, +4 and +6. Sulfur forms stable compounds with all elements except the noble gases. One of the basic ingredients for many fireworks. Flowers of sulphur is too acidic and if used need a stabilizer added. Sulphur and chlorates or phosphorous are extremely shock and friction sensitive and should be avoided. Used primarily with nitrates. Sulfur has always been used extensively in pyrotechnics. It serves as a fuel, and reduces the ignition temperature of mixtures. It also tends to increase the burning rate and friction or shock sensitivity of most mixtures. |
Sulfur can increase the sensitivity of some mixtures, especially those based on chlorate or perchlorate oxidizers. Mixtures of chlorates and sulfur are also known to ignite spontaneously and should therefore be avoided at all times (also read the safety section). Mixtures of perchlorates and sulfur are less likely to ignite spontaneously but are still very sensitive and need to be treated with extreme caution. Burning sulfur produces sulfur dioxide gas, inhalation of which should be avoided because it is extremely poisonous. | Sulfur is available from agricultural supply stores where it is sold as a fungicide under the name 'dusting sulfur'. It is a fine powder mixed with a few percent of calcium carbonate. The calcium carbonate may disturb delicate color compositions, but for most purposes dusting sulfur works well. If a purer form of sulfur is required, sulfur may also be obtained from drug stores sometimes. However, these often sell 'flowers of sulfur', which has been purified by sublimation and which contains some acid. This needs to be neutralized before use as it could cause spontaneous ignition. To do this, allow 100g of this sulfur to soak in a liter of water/household ammonia (1:5). Stir well occasionally and measure the pH. It should still be alkaline after two days, after which time the sulfur may be filtered and washed with hot water to remove the ammonia. Check the pH of the washing water while filtering. After it has become neutral, flush the water away with ethanol and allow the sulfur to dry. Mix the dry powder with 2% magnesium carbonate to neutralize any acid that may be formed in reactions with the atmosphere. Elemental sulfur can be found near hot springs and volcanic regions in many parts of the world, especially along the Pacific Ring of Fire. Such volcanic deposits are currently exploited in Indonesia, Chile, and Japan. Significant desposits of elemental sulfur also exist in salt domes along the coast of the Gulf of Mexico, and in evaporites in eastern Europe and western Asia. The sulfur in these deposits is believed to come from the action of anaerobic bacteria on sulfate minerals, especially gypsum, although apparently native sulfur may be produced by geological processes alone, without the aid of living organisms (see below). However, fossil-based sulfur deposits from salt domes are the basis for commercial production in the United States, Poland, Russia, Turkmenistan, and Ukraine. Sulfur, produced in the hydrodesulfurization of oil, gas and the Athabasca Oil Sands has become a glut on the market, with huge stockpiles of sulfur in existence throughout Alberta. |
Fuel | ||
Titanium Ti Flake titanium is often added to salutes and other formulations to add silver-blue sparks. Metallic titanium is used in pyrotechnics to produce bright white sparks that are known to branch out in a unique, fine-grain structure. The particle size and shape of the titanium will affect the color and duration of the sparks, with smaller mesh producing smaller and shorter-lasting sparks. |
Fuel | Silver | |||
Xylene Solvents that are used for parlon, saran, and paint thining etc. They are also used to solvent-bond plastic aerial shell halves. |
Xylene affects the brain. High levels from exposure for short periods (14 days or less) or long periods (more than 1 year) can cause headaches, lack of muscle coordination, dizziness, confusion, and changes in one's sense of balance. Exposure of people to high levels of xylene for short periods can also cause irritation of the skin, eyes, nose, and throat; difficulty in breathing; problems with the lungs; delayed reaction time; memory difficulties; stomach discomfort; and possibly changes in the liver and kidneys. It can cause unconsciousness and even death at very high levels (often seen similar to huffing). | These chemicals can easily be purchased from paint and hardware stores. It can not be made at home. | |||
Zinc Zn Sensitive to moisture and spontaneous combustion. Metallic zinc is used in rocket propellants, for spark effects and in white smoke compositions. Zinc powder is quite heavy and zinc-based stars often require heavier lift or burst charges to propel them. Rarely used anymore as a primary fuel but sometimes used as a secondary enhancement fuel. Zinc is a moderately reactive bluish-white metal that tarnishes in moist air and burns in air with a bright greenish flame, giving off plumes of zinc oxide. It reacts with acids and alkalis and other non-metals. If not completely pure, zinc reacts with dilute acids to release hydrogen. The one common oxidation state of zinc is +2. From 100 °C to 210 °C zinc metal is malleable and can easily be beaten into various shapes. Above 210 °C, the metal becomes brittle and will be pulverized by beating. |
Zinc powder can spontaneously heat up when wet. Metallic zinc is not considered to be toxic, but free zinc ions in solution (like copper or iron ions) are highly toxic. There is also a condition called zinc shakes or zinc chills (see metal fume fever) that can be induced by the inhalation of freshly formed zinc oxide formed during the welding of galvanized materials. Excessive intake of zinc can promote deficiency in other dietary minerals. | Zinc powder is used in paints for the protection of steel. Spray cans containing an suspension of zinc powder are commonly sold in hardware stores. The zinc powder may be extracted by emptying the spray can in a large container, allowing the powder to settle, decanting the solvent and paints and repeated washing with paint thinner or acetone. However this is an expensive way to obtain zinc powder when high purity is available commercially at about $3 per pound in 50 pound drums. Unfortunately it cakes to rock hard masses in a few years on storage and requires ball milling every year to keep it usable. | Fuel, color agent | Green, Smokes | 590nm |
Zinc Oxide ZnO Zinc oxide is used to produce white smoke. |
Zinc oxide is not particularly toxic or dangerous. | Zinc oxide is usually available as a white pigment called 'zinc white' in artistic paint stores. It can also be prepared by igniting a piece of zinc sheet. | Fuel | Smokes and flares | |
| Table based on these sources: http://www.wfvisser.dds.nl/EN/cheminfo_EN.html http://en.wikipedia.org/wiki/Category:Pyrotechnic_chemicals http://www.pyro-pages.com/Info/Chemistry/chemical.htm http://www.pyroguide.com/index.php?title=Category:Chemicals http://scifun.chem.wisc.edu/CHEMWEEK/fireworks/fireworks.htm |
Generally speaking, the various chemicals used in firework manufacture can be subdivided into the following categories: Oxidizing Agents: Oxidizers produce oxygen to burn the mixture. Oxidizers are usually nitrates, chlorates, or perchlorates.(eg Potassium Nitrate) Fuels: (eg charcoal) Color Agents: Essential to produce specific colors Binding Agents: Binders hold the mixture together. For a sparkler, common binders are dextrin (a sugar) dampened by water, or a shellac compound dampened by alcohol. The binder can serve as a reducing agent and as a reaction moderator. (eg dextrin) act as a ‘glue’ to hold chemicals together |
 |
|||
Home ►◄ Pyrotechnic Links ►◄ Pyrotechnic Files ►◄ Firework Chemicals ►◄ Pyrotechnics Data ►◄ Site Map |
|||||
|
|||||